Periodic Table Everything You Need to Know
Everything Yous Demand To Know About The Periodic Table

The periodic tabular array is one of those classic images that you lot find in many science labs and classrooms. It's an image almost everyone has seen at some time in their life.
You can too notice the periodic table on t-shirts, mugs, embankment towels, pillowcases and duvet covers, and plenty of other items. It even inspired a collection of brusque stories.
Who can forget the periodic table put to music by the American Tom Lehrer, a Harvard mathematics professor who was also a singer/songwriter and satirist. His vocal, The Elements, includes all the elements that were known at the fourth dimension of writing in 1959.
Since then, several new elements have been added to the periodic table, including the four that were formally approved last yr by the International Marriage of Pure and Practical Chemistry (IUPAC).
But what exactly does the periodic table prove?
In brief, it is an attempt to organise the collection of the elements – all of the known pure compounds made from a single type of cantlet.
There are two ways to look at how the periodic table is constructed, based on either the observed backdrop of the elements contained within it, or on the subatomic construction of the atoms that form each element.
Shutterstock/duntaro
The elements
When scientists began collecting elements in the 1700s and 1800s, slowly identifying new ones over decades of research, they began to notice patterns and similarities in their physical properties. Some were gases, some were shiny metals, some reacted violently with h2o, and and then on.
At the time when elements were first being discovered, the structure of atoms was not known. Scientists began to look at ways to adapt them systematically then that like backdrop could be grouped together, just as someone collecting seashells might try to organise them by shape or color.
The task was made more difficult because not all of the elements were known. This left gaps, which made deciphering patterns a bit like trying to assemble a jigsaw puzzle with missing pieces.
Different scientists came up with different types of tables. The kickoff version of the current table is mostly attributed to Russian chemistry professor Dmitri Mendeleev in 1869, with an updated version in 1871.
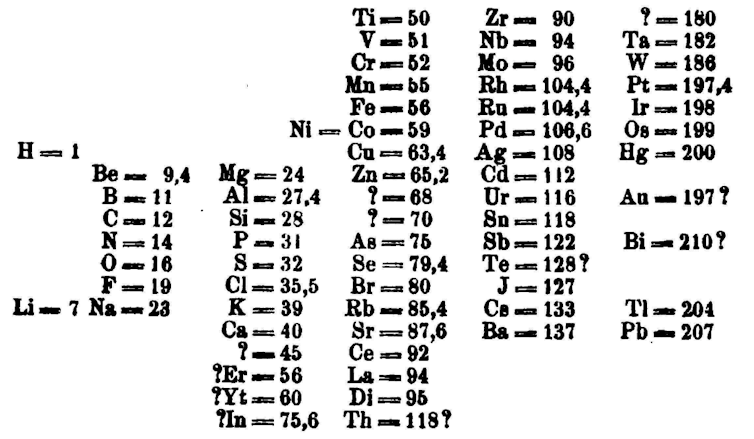
Mendeleev's periodic table is first published outside Russian federation in Zeitschrift für Chemie (1869, pages 405-6).
(Wikimedia/Dimitri Mendeleev)
Chiefly, Mendeleev left gaps in the table where he thought missing elements should be placed. Over time, these gaps were filled in and the concluding version equally we know it today emerged.
The atoms
To really understand the last structure of the periodic tabular array, we demand to empathise a flake about atoms and how they are constructed. Atoms take a primal core (the nucleus) made up of smaller particles called protons and neutrons.
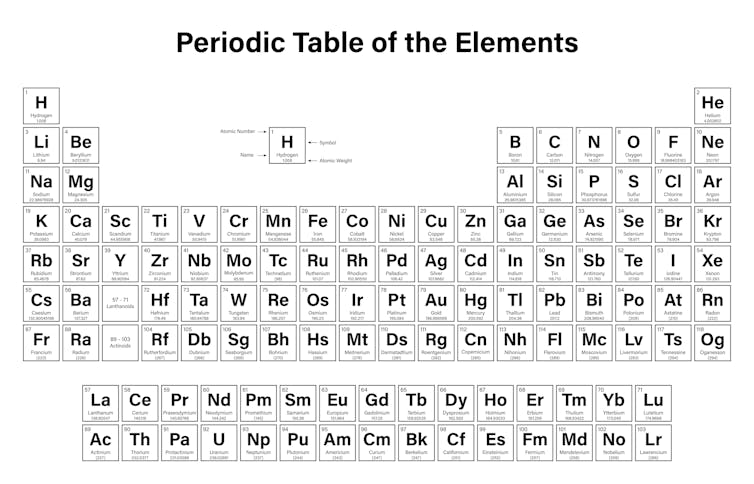
It is the number of protons that gives an chemical element its atomic number – the number generally found in the elevation left corner of each box in the periodic table.
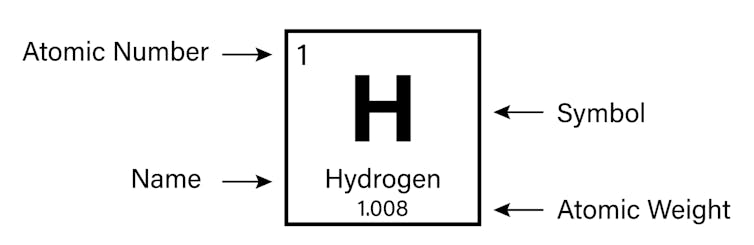
The properties of hydrogen as marked on the periodic table.
(Shutterstock/duntaro)
The periodic table is arranged in club of increasing atomic number (left to correct, top to lesser). It ranges from chemical element 1 (hydrogen H) in the acme left, to the newly canonical chemical element 118 (oganesson Og) in the bottom right.
The number of neutrons in the nucleus tin vary. This gives rise to different isotopes for every element.
For case, you may accept heard of carbon-14 dating to determine the historic period of objects. This isotope is a radioactive version of normal carbon C (or carbon-12) that has two extra neutrons.
But why is at that place a separate box of elements beneath the main table, and why is the main table an odd shape, with a seize with teeth taken out of the top? That comes downwardly to how the other component of the atom – the electrons – are arranged.
The electrons
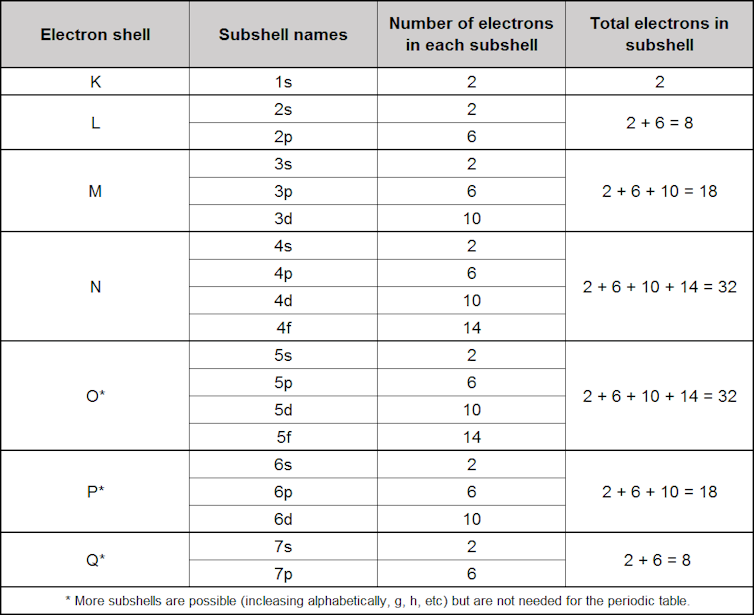
We tend to think of atoms as built a bit similar onions, with 7 layers of electrons chosen "shells", labelled Thou, L, Thousand, N, O, P, and Q, surrounding the cadre nucleus.
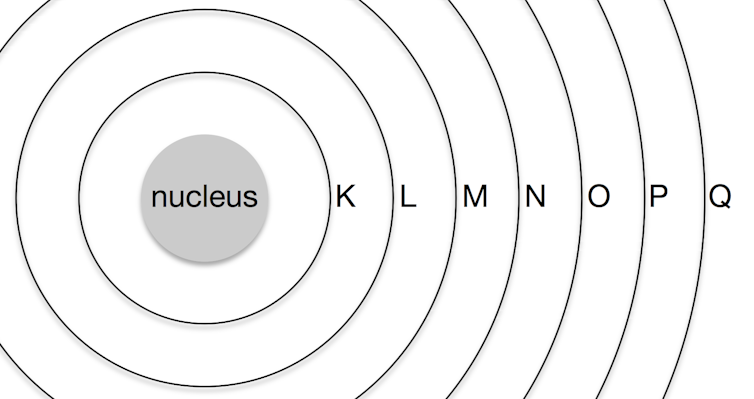
Retrieve of the atom with a central nucleus that contains all the protons and neutrons, surrounded past a series of shells that contain the electrons.
(The Conversation, CC BY-ND)
Each row in the periodic table sort of corresponds to filling up one of these shells with electrons. Each shell has subshells, and the social club in which the shells/subshells get filled is based on the energy required, although it's a complicated process. We'll come dorsum to these afterward.
In simple terms, the first element in each row starts a new crush containing one electron, while the last element in each row has 2 (or one for the the first row) of the subshells in the outer shell fully occupied. These differences in electrons likewise account for some of the similarities in properties between elements.
With the one or two subshells in the outer layer total of electrons, the concluding elements of each row are quite unreactive, as there are no holes or gaps in the outer trounce to interact with other atoms.
This is why elements in the concluding column, such every bit helium He, neon (Ne), argon (Ar) and so on, are called the noble gases (or inert gases). They are all gases and they are "noble" considering they rarely associate with other elements.
In contrast, the elements of the first column, with the exception of hydrogen (just like English grammar, in that location's ever an exception!), are called alkali metals. The offset-column elements are metal-similar in character, but with only one electron in the outer shell, they are very reactive as this lone electron is very piece of cake to engage in chemic bonding. When added to water, they speedily react to form an alkaline metal (basic) solution.
Each shell can arrange an increasing number of electrons. The first shell (1000) only fits two, so the first row of the periodic table has just ii elements: hydrogen (H) with one electron, and helium (He) with ii.
The 2d trounce (L) fits eight electrons. Thus the 2d row of the periodic table contains eight elements, with a gap left between hydrogen and helium to adapt the extra six.
The third beat (1000) fits 18 electrons, but the third row nevertheless only has 8 elements. This is because the actress ten electrons don't get added to this layer until after the first two electrons are added to the fourth shell (N) (nosotros'll get to why, later).
So the gap is expanded in the fourth row to accommodate the boosted ten elements, leading to the "bite" out of the height of the table. The actress 10 compounds in the center section are called the transition metals.
The fourth crush holds 32 electrons, only over again the actress electrons are not added to this shell until some have also been added to the fifth (O) and sixth (P) shells, meaning that both the fourth and 5th rows concur 18 elements.
For the side by side ii rows (sixth and seventh), rather than further expanding the table sideways to include these actress xiv elements, which would brand it too wide to easily read, they have been inserted as a block of ii rows, called the lanthanoids (elements 57 to 71) and actinoids (elements 89 to 103), below the main table.
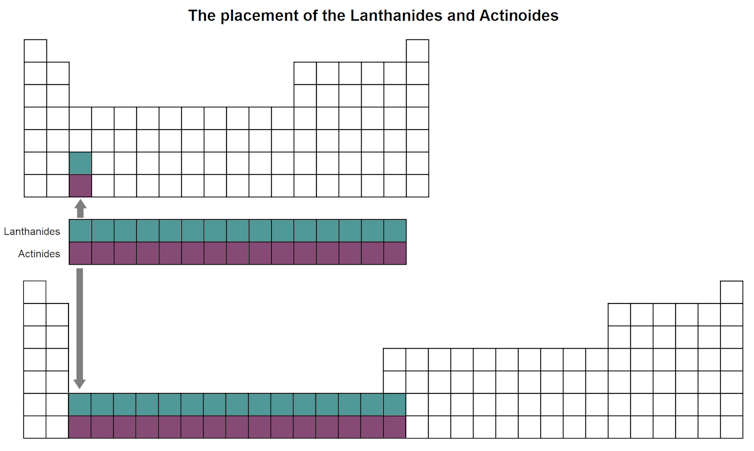
The periodic table would look very unlike if the lanthanoids and actinoids were inserted inside the table.
(The Chat, CC Past)
You can see where they would fit in if the periodic table was widened, if y'all look at the lesser 2 squares in the third column of the table above.
Across the columns
There is another complicating cistron leading to the final shape of the table. As mentioned earlier, as the electrons are added to each layer they go into different subshells (or orbitals), which describes locations effectually the nucleus where they are most likely to be constitute. These are known by the letters s, p, d and f.
The messages used for the orbitals are actually derived from descriptions of the emission or absorption of lite due to electrons moving between the orbitals: sharp, principal, diffuse and fundamental.
Each shell has its own configuration of subshells named from 1s through to 7p, which gives the full number of electrons in each shell as we progress through the periodic table.
(The Conversation, CC By-ND)
Equally mentioned earlier the club in which the subshells fill up with electrons is not so straightforward. Yous can come across the guild in which they fill from the image below, just follow the order every bit you lot would read down from left to correct.
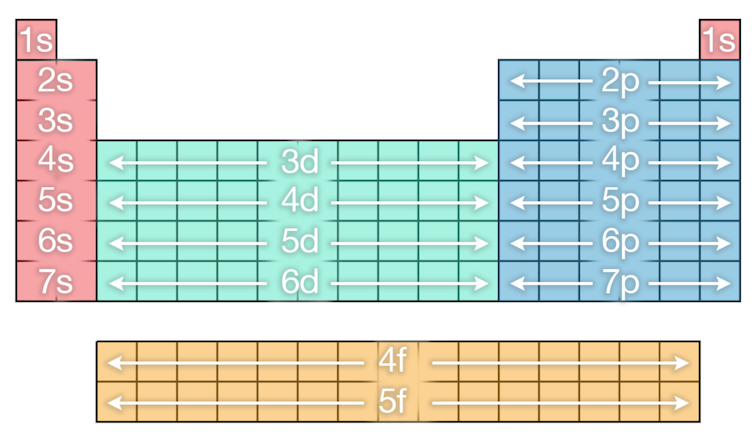
(The Conversation, CC Past-SA)
There is an interactive periodic tabular array that too illustrates the filling sequence well if you click through the atoms.
Elements within a cavalcade generally have similar properties, merely in some places elements side by side can also exist similar. For instance, in the transition metals the cluster of precious metals effectually copper (Cu), silverish (Ag), aureate (Au), palladium (Pd) and platinum (Pt) are quite alike.
Most of the existing elements with loftier atomic numbers, including the four superheavy elements added last twelvemonth, are very unstable and have never been detected in, or isolated from, nature.
Instead, they are created and analysed in minute quantities under highly bogus conditions. Theoretically, there could be further elements beyond the 118 now known (there are boosted yard, h and i suborbitals), merely we don't know yet if any of these would be stable plenty to exist isolated.
A classic design
The periodic table has seen many colourful and informative versions created over the years.

The Periodic table decorates a taxi in the United kingdom of great britain and northern ireland.
(Flickr/Fr Gaurav Shroff, CC BY-NC-ND)
Ane of my favourites is an artistic version with original artworks for each element commissioned past the Royal Australian Chemic Found to celebrate the International Year of Chemistry in 2011.
Another favourite is an interactive version with pictures of the elements. The creators of this site have also published a java table book called The Elements and an Apple app with videos of each element.
Interactive versions have as well been created past the Royal Society of Chemical science (and can also be downloaded as an app) and ChemEd DL amid others.
The classic design of the periodic table can be used to play a version of the Battleship game.

Playing battleships with the periodic tabular array at the outset World Science Festival Brisbane in 2016.
(The Conversation, CC BY-NC-ND)
In that location are as well many fun versions created to assist organise a multitude of objects, including food, beer, emojis, iPad apps and birds.
As for Tom Lehrer'south The Elements, the song has withal to be updated to include all the elements known today but it has been covered by other people over the years.
Actor Daniel Radcliffe, of Harry Potter fame, performed a version during a guest advent on the BBC'due south Graham Norton Show.
There are other musical versions of the elements simply they too take yet to exist updated to include all entries of the periodic table.
In summary, the periodic table is the chemist's taxonomy of all elements. Its triumph is that information technology is nonetheless highly relevant to scientists, while likewise becoming embedded in popular culture. 
Mark Blaskovich, Senior Inquiry Officer, The Academy of Queensland
This article is republished from The Conversation under a Creative Commons license.
More From Lifehacker Australia
Source: https://www.lifehacker.com.au/2020/02/the-periodic-table-explained/
Enviar um comentário for "Periodic Table Everything You Need to Know"